Background. Treatment of newly diagnosed multiple myeloma (NDMM) consists of induction therapy followed by autologous stem cell transplant (ASCT) and indefinite maintenance. Continuous lenalidomide carries both significant clinical and financial toxicity. Minimal residual disease (MRD) has proven to be a powerful surrogate for PFS and OS but is not yet used to guide treatment. We hypothesize that MRD could be used to predict a subset of patients who can safely stop maintenance therapy and enter into a disease surveillance phase.
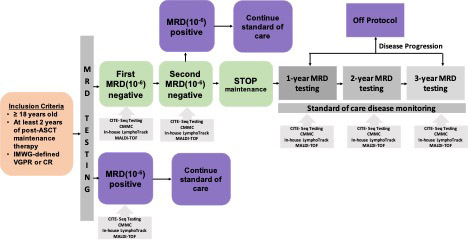
Study Design and Methods. This prospective phase 2 study (NCT 05192122) is designed to stop post-ASCT maintenance therapy once sustained MRD negativity is achieved. MM patients are eligible if they have completed ≥2 years of maintenance therapy post-ASCT, and meet IMWG criteria for ≥ PR. Bone marrow (BM) MRD testing is performed twice, one year apart, using NGS. Sustained MRD-negativity is defined at a threshold of 10 -6, with negative MRD sustained between the Year 1 and Year 2 interval. Patients with sustained MRD negativity and absence of new bony lesions on PET then undergo maintenance therapy cessation. MRD-positive patients are removed from study. In patients who discontinue maintenance, BM testing to re-assess for MRD is repeated yearly for 3 years. At the time of MRD testing, peripheral blood (PB) samples are collected for MALDI-TOF mass spectrometry testing and circulating multiple myeloma cell (CMMC) enumeration. These are done in parallel with MRD assessment. Health-related quality of life (HRQoL) using the EORTC QLQ-MY20 questionnaire is measured before and after maintenance cessation. This study plans to enroll 50 patients to meet the primary objective of assessing sustained MRD-negativity 1 year after maintenance cessation. For all patients, 5-mL PB and BM samples are collected, processed, and kept frozen until analysis. Cellular indexing of transcriptomes and epitopes (CITE-seq) to simultaneously quantify cell surface protein and transcriptome data is used to characterize differences in the immune microenvironment between MRD-negative, MRD-positive, as well as between patients with biochemical and/or clinical progression and patients with MRD-resurgence but biochemically and clinically stable.
Preliminary Results. FREEDMM enrollment began December 2021, and 31 patients have been enrolled, 29 of whom have undergone Year 1 BM for MRD assessment. Median follow up of our cohort is 414 (0-577) days. The median age is 69.5 (40-78) years, and the majority are either Black (n=9; 29%) or Hispanic (n=11; 35%). 18 (58%) patients had R-ISS 1 or 2, while 2 patients had high risk disease with presence of 17p deletion. The median number of years of maintenance therapy completed prior to Year 1 BM is 3.4 (2-7.9) years. 28 (96.6%) were in CR at time of Year 1 BM. Year 1 MRD results are pending in 13 patients. At Year 1, 11 (68.8 %) of 16 patients were found to be MRD-negative, while 5 (31.3%) patients were found to be MRD-positive with a median of 206 (1-9,748) cells per 10 6 nucleated cells detected. 24 (85%) of 28 patients had a negative MALDI-TOF result at the time of the Year 1 BM. MALDI-TOF results were false negative in 3 MRD-positive patients. PB CMMC samples were concordant with MRD results in all 5 (100%) patients who were tested. There was concordance between MALDI-TOF and CMMC results in 9 (75%) of 12 patients. At year 2, 4 patients continued to be MRD negative, while 1 patient who was previously MRD-negative became MRD positive. At most recent follow-up, 3 (42.9%) of 7 MRD positive patients at Year 1 experienced clinical progression and were removed from the study. 2 (50%) of 4 patients with sustained MRD-negativity have undergone maintenance cessation, with the remaining 2 awaiting confirmation of MRD-negative results. 59 paired PB (n=29) and BM (n=30) samples have been collected for CITE-seq analysis. Pilot CITE-seq experiments captured 10,000-15,000 cells with 33,000-56,000 reads/ cell on one MRD-positive BM and paired PB sample. CITE-seq results for remaining patients will be presented at the conference.
Conclusion: This phase 2 trial applies a novel clinical trial design with an adaptive strategy to explore use of MRD testing as a guide to maintenance therapy cessation in MM patients and represents one of the first studies introducing transcriptome analysis of the tumor and immune microenvironment in the maintenance setting. Ongoing results will be reported at the conference.
Disclosures
Hofmeister:BMS: Research Funding; Pfizer: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees. Quigley:Mitsubishi: Consultancy; Pfizer: Research Funding; Rigel Pharmaceuticals Inc.: Current equity holder in publicly-traded company, Honoraria; Teva Pharmaceuticals: Research Funding; Alnylam Pharmaceuticals: Speakers Bureau; Servier Pharmaceuticals: Speakers Bureau; Amgen Pharmaceuticals: Research Funding; Recordati Rare Diseases, Inc: Honoraria; AbbVie: Research Funding. Sborov:Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Arcellx: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Adaptive Biotech: Other: Payment for an educational seminar; Bristol Myer Squibb: Membership on an entity's Board of Directors or advisory committees; BinayTara Foundation: Other: Support for attending meetings and/or travel; GSK: Consultancy. Rondelli:Vertex: Other: Steering Committee. Sweiss:Sanofi: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal